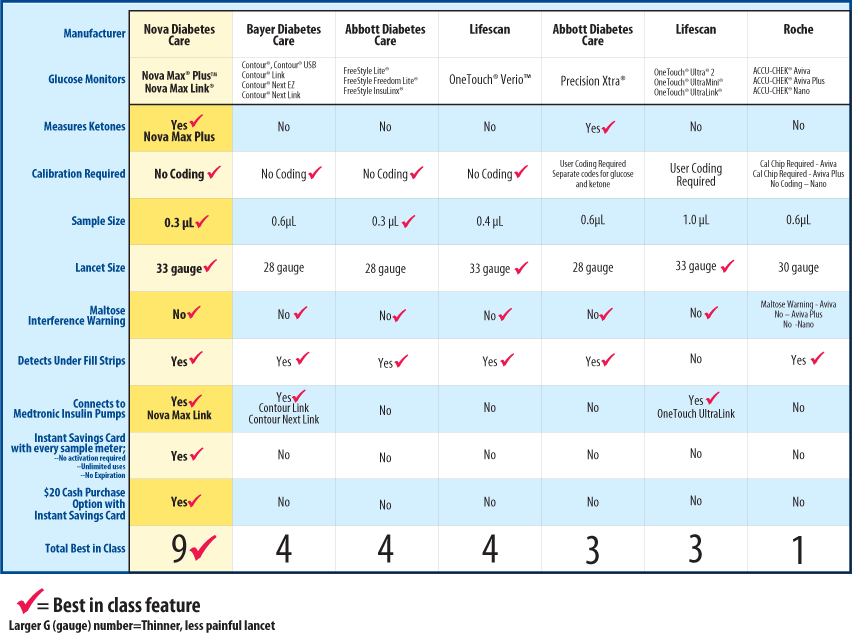
Blood Glucose Meter 2010Working Group Report By The Working Group on Medical Measurements The Seventeenth Forum Meeting October 14-16, ppt download
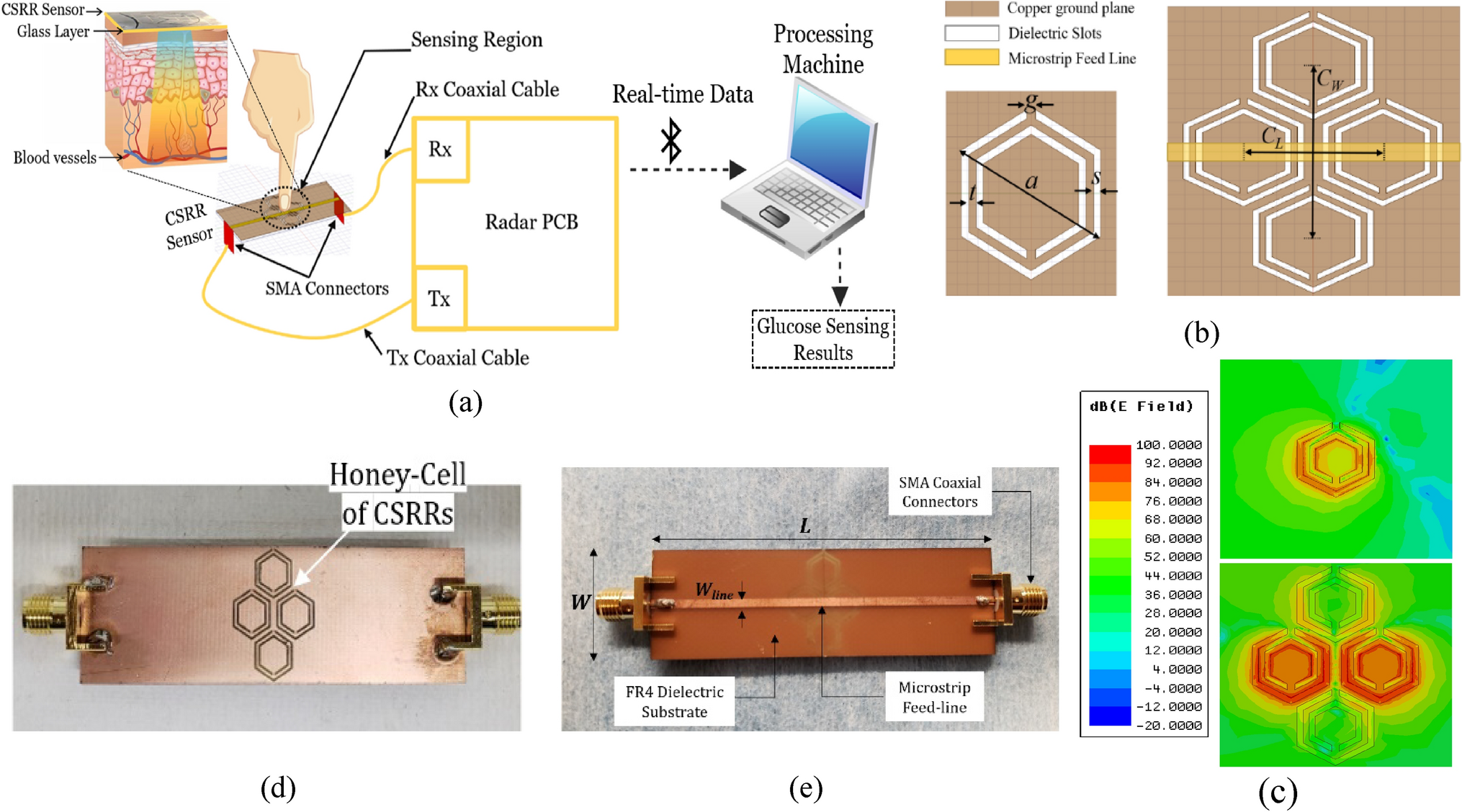
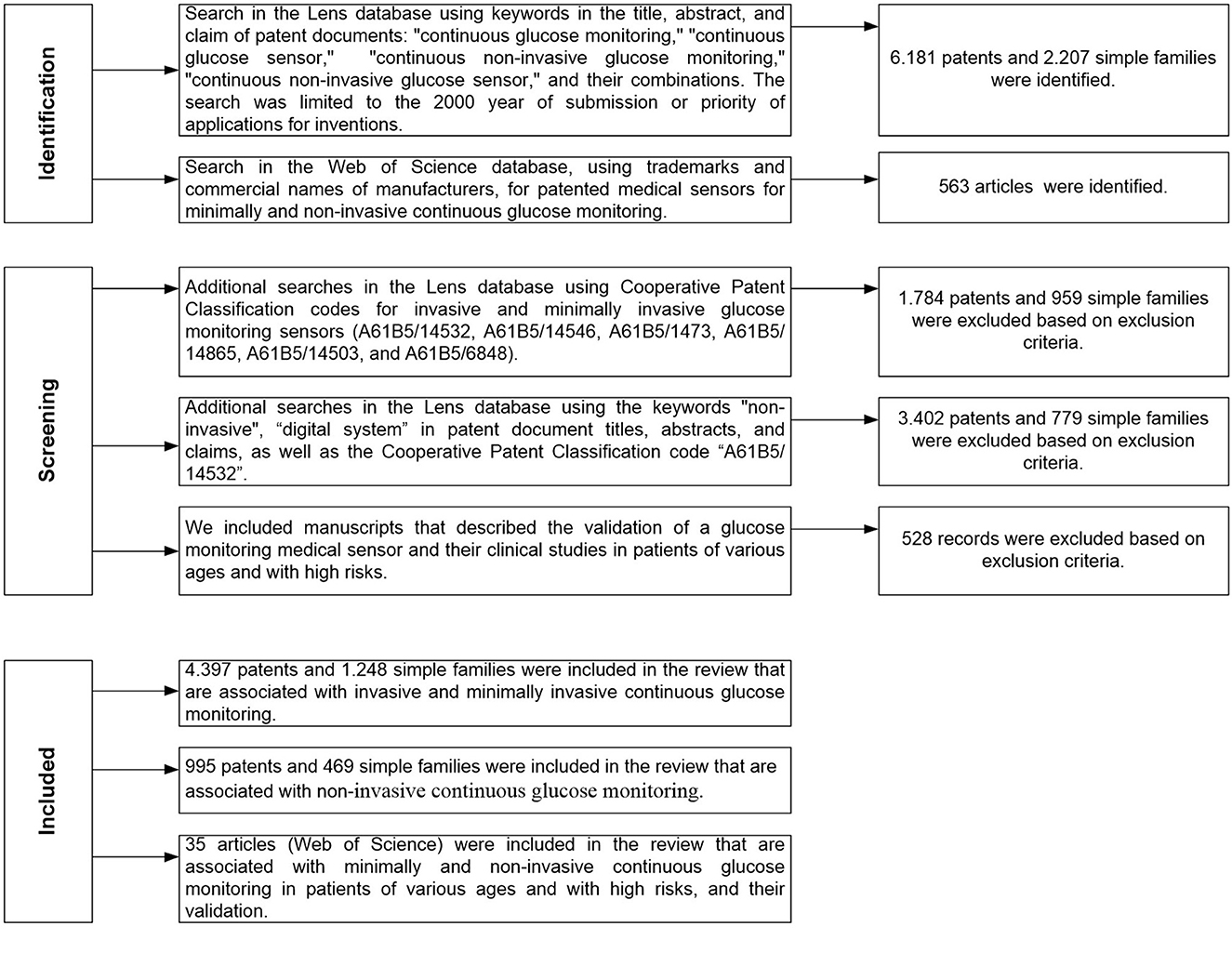
Low-cost portable microwave sensor for non-invasive monitoring of blood glucose level: novel design utilizing a four-cell CSRR hexagonal configuration | Scientific Reports

Clinical FDA CE Blood Sugar Detection Bluetooth Automatic Blood Glucose Monitor with Test Strips Transmission Protocol - China Glucometer, Glucose Meter | Made-in-China.com

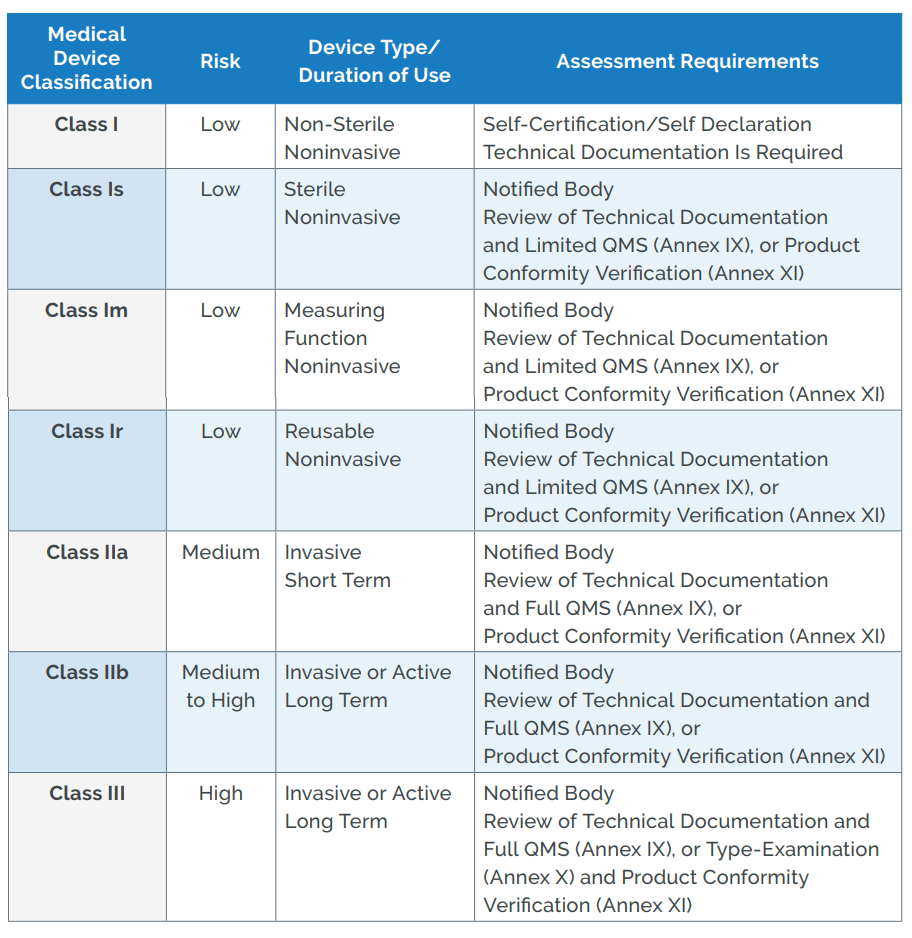
Glucose meters: current regulatory guidance for manufacturers and providers | Medical Laboratory Observer

Glucose meters: current regulatory guidance for manufacturers and providers | Medical Laboratory Observer

Glucose meters: current regulatory guidance for manufacturers and providers | Medical Laboratory Observer
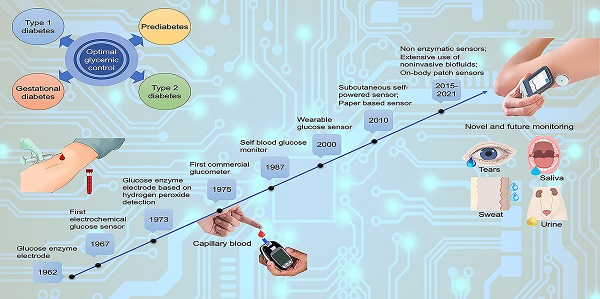
Glucose biosensors in clinical practice: principles, limits and perspectives of currently used devices
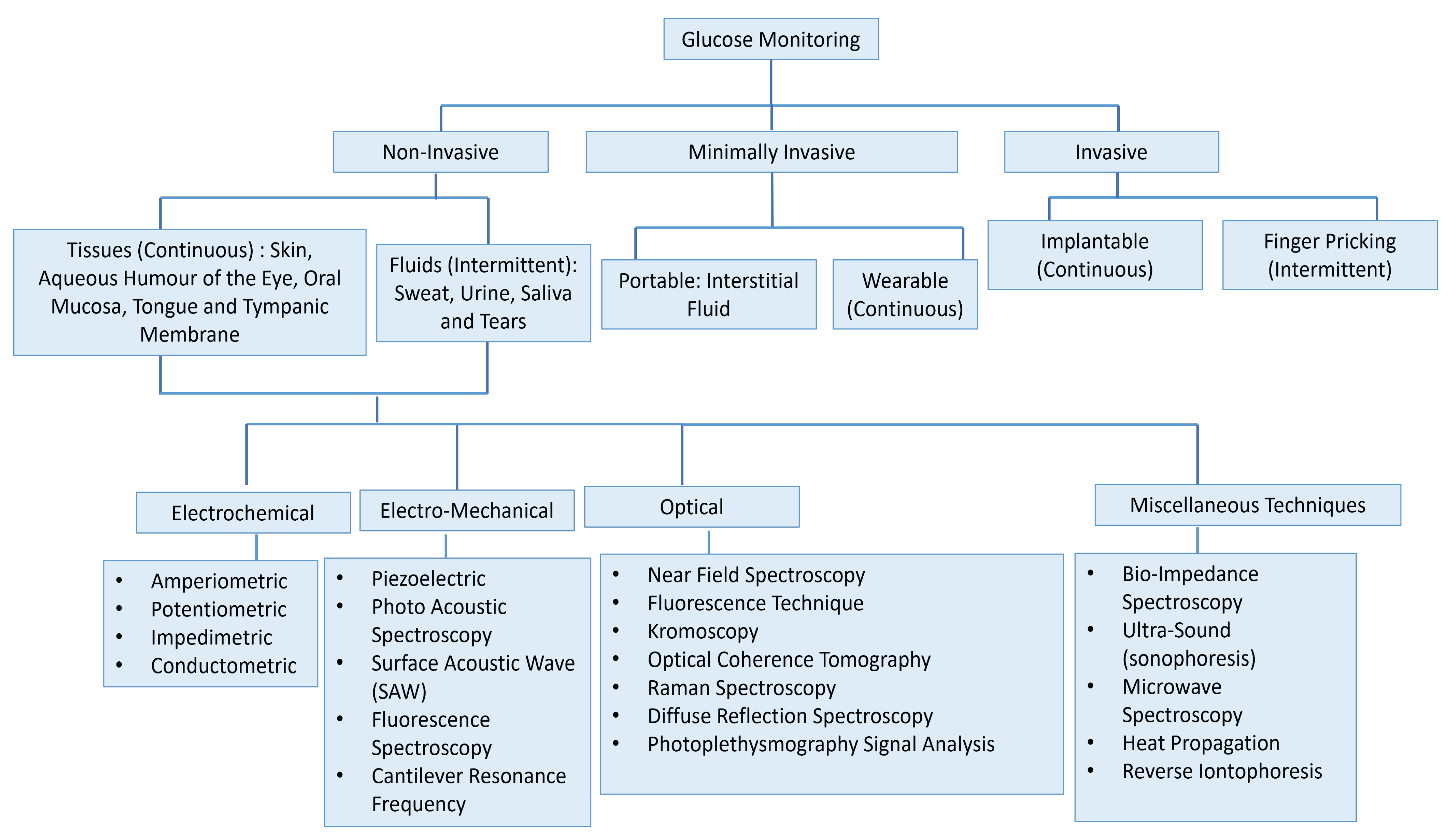
Sensors | Free Full-Text | Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring

FDA Issues Final Orders to Reclassify Blood Lancet Devices into Class II and Class III Devices; Orders Include a PMA Requirement for Class III Blood Lancets - Registrar Corp

PRECLINICAL TESTING - Expanding Opportunities in Implantable Medical Devices With Optimized Preclinical Studies
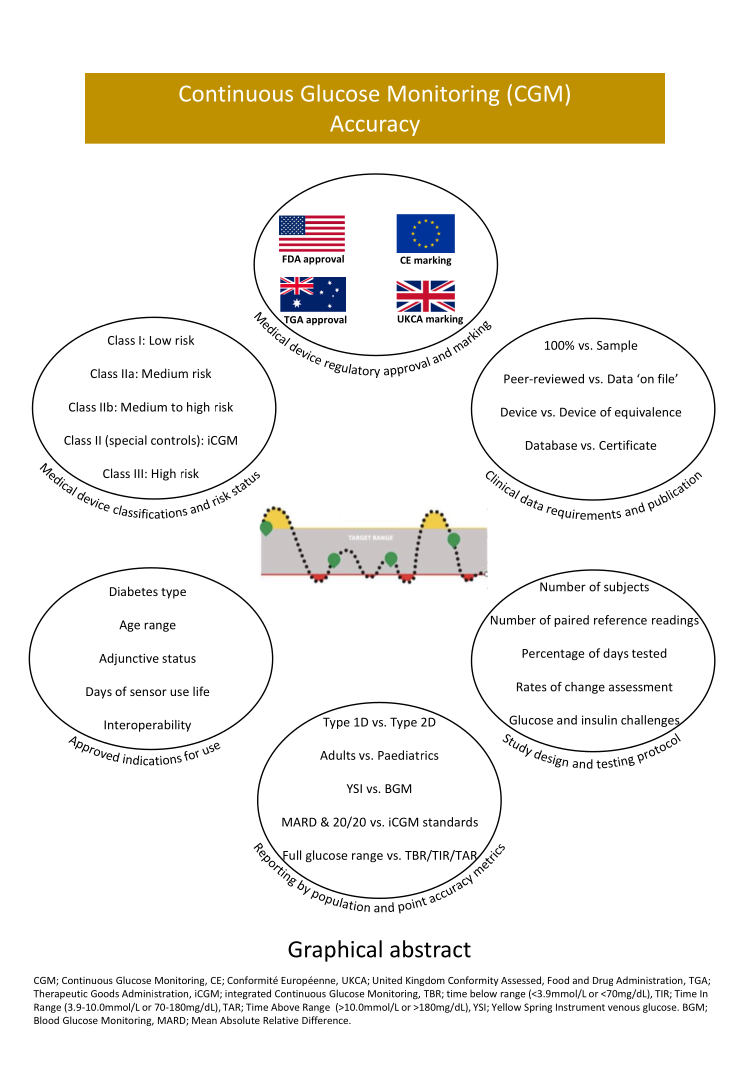
CGM accuracy: Contrasting CE marking with the governmental controls of the USA (FDA) and Australia (TGA): A narrative review - Pemberton - 2023 - Diabetes, Obesity and Metabolism - Wiley Online Library

Glucose meters: current regulatory guidance for manufacturers and providers | Medical Laboratory Observer